FDA Expectations for Real-World Imaging Data: What Makes RWE Regulatory-Grade

Reading time /
3 min
Industry


Real-world evidence (RWE) built from real-world imaging data (RWiD) is becoming a cornerstone of healthcare innovation. Routine PACS (Picture Archiving and Communication System) images such as x-rays, CT, MRI, and ultrasound exams are now recognized as a powerful window into patient outcomes, treatment response, and disease biology. From regulatory decisions and clinical trials to AI development and post-market surveillance, imaging data is rapidly becoming one of the strongest real-world evidence sources.
As RWiD applications continue to expand, the FDA is sharpening its expectations around how imaging-derived RWE should be collected, curated, and validated to achieve true regulatory-grade credibility.
Imaging Data Matters - For Research, Patient Care, and Regulation
Imaging data is crucial to research and advancing patient care because it provides objective, reproducible, and detailed evidence of how diseases evolve and how therapies work in the body. This importance is also recognized by regulators, who are focused on ensuring such data is used correctly and consistently in guiding decisions.
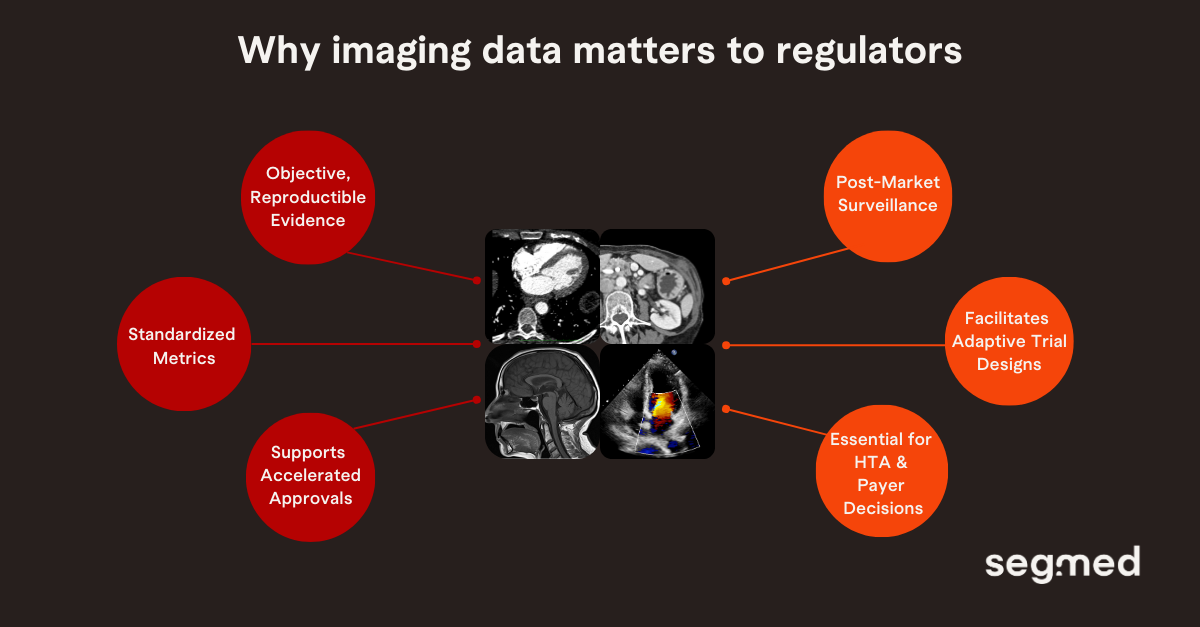
Imaging data enables surrogate endpoints that accelerate approvals, as seen with Alzheimer’s drugs like Lecanemab and Donanemab via early amyloid and tau PET, and in oncology through CT- or MRI-based endpoints like objective response rate and progression-free survival.
Beyond early approvals, it supports post-marketing surveillance by confirming real-world durability in therapies such as multiple sclerosis, CAR-T, and liver disease, while also informing Health Technology Assessments (HTA) and payer assessments of long-term effectiveness and economic value.
With AI diagnostics and digital biomarkers rising, real-world imaging data will be key for validating algorithms, detecting bias, and guiding regulatory decisions from early approval to long-term access.
The FDA’s North Star: “Fit-For-Purpose” Evidence
FDA’s evolving guidance on RWE consistently points to one overarching principle:
“Evidence must be fit-for-purpose, meaning, appropriate, reliable and rigorously generated for the specific regulatory question it aims to answer.”

For imaging-derived RWE, the FDA evaluates how imaging is sourced, curated, standardized, and analyzed, as even minor inconsistencies can affect outcomes. The agency encourages early engagement and requires detailed protocols covering sources, acquisition parameters, harmonization, and analytics. Submissions must clearly show how the imaging supports regulatory objectives, whether for safety, effectiveness, surrogate endpoints, or post-market insights.
FDA programs such as the Advancing RWE Initiative and RWE Demonstration Projects define high-quality imaging evidence, providing transparency and shared learnings to industry. This establishes the foundation for regulatory-grade RWiD data that is both scientifically sound and operationally reliable. The next step is identifying the core elements that ensure imaging data can withstand inspection, support claims, and meaningfully guide regulatory decisions.
Practical Checklist For RWiD Submission
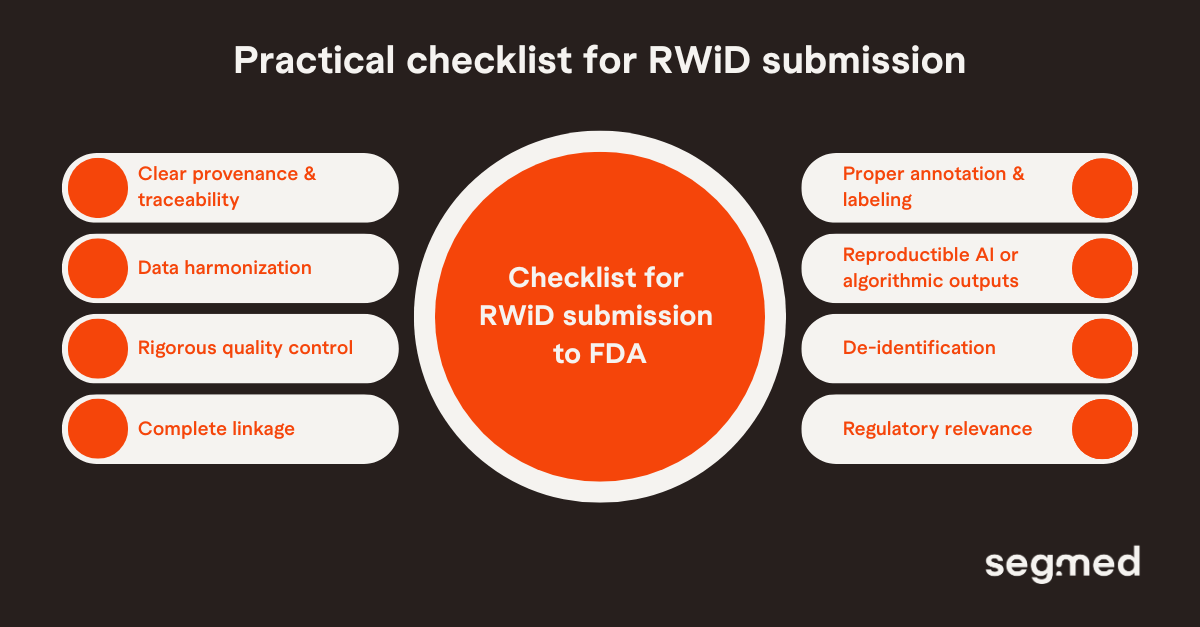
Regulatory-grade RWiD is not just aggregated scans; it requires precision, consistency, and transparency on par with clinical trial imaging. As the FDA increasingly evaluates imaging-derived RWE, certain foundational elements have emerged as essential markers of quality. These elements ensure that imaging data is rich and clinically meaningful and also reliable enough to support decisions that impact patient safety and therapeutic value.
1. Traceable and Verifiable Data Provenance
Regulators expect full visibility into where the imaging data originated, how it was captured, and how it moved through each stage of the data pipeline. This includes scanner details, acquisition parameters, site-level variability, and audit trails documenting any transformations.
2. Standardized Acquisition and Harmonization
Owing to significant variability in imaging practices across institutions, regulatory-grade RWiD requires strong harmonization.Techniques such as protocol standardization, normalization, and quality control checks help ensure that images collected across different scanners, sites, and time periods are comparable.
3. High-Quality Annotation and Curation
The FDA places significant emphasis on how images are labeled, segmented, and interpreted. Whether annotations come from US board certified radiologists, automated tools, or hybrid workflows, they must be accurate, reproducible, and supported by quality assurance procedures.
4. Robust Data Transformation and Feature Extraction
Imaging datasets often undergo significant processing, especially when used to derive quantitative biomarkers or AI features. Each transformation such as denoising, reconstruction, segmentation, radiomics extraction must be validated and documented. Regulators expect transparency around the entire analytic pipeline to ensure the results are scientifically sound and reproducible.
5. Privacy-Preserving yet Information-Rich De-identification
Imaging data often includes patient-identifiers, requiring regulatory-grade RWiD to implement advanced de-identification pipelines that preserve diagnostic and quantitative integrity. Approaches such as metadata scrubbing and structured safe harbor practices ensure compliance without sacrificing analytic value.
6. Operational Quality Controls at Scale
Large-scale imaging datasets amplify risks such as scanner drift, labeling inconsistencies, and missing slices. Continuous monitoring, periodic QC audits, reproducibility checks, and automated anomaly detection help preserve dataset integrity over time, a standard consistently reinforced in the FDA’s RWE guidance.
7. Clear Linkage to Clinical Context
Regulatory grade imaging data must be clearly connected to clinical context, including the patient’s diagnosis, treatment details, outcomes, and the point in the care pathway. Regulators look for this contextual completeness because it helps them interpret what the image actually represents, reduces confounding, and supports stronger conclusions about treatment effects.
8. Transparency, Documentation, and Audit Readiness
Finally, regulatory-grade RWiD requires meticulous documentation. From data dictionaries and SOPs to protocol deviations and algorithm version histories, every step must be auditable. This level of transparency builds trust and provides regulators with the confidence needed to rely on imaging-derived RWE for high-stakes decisions.
Closing Perspective
The FDA’s expectations for imaging-derived RWE reflects a balance between innovation and scientific rigor. As imaging data increasingly shapes regulatory and market access decisions, its quality, standardization, and transparency become essential. The agency’s emphasis on fit-for-purpose data, methodological clarity, and representative validation sets a high standard that continues to evolve through programs like the Advancing RWE Initiative.
For sponsors and researchers, this environment is not a barrier but an invitation to build stronger datasets. Imaging data that is well sourced, harmonized, clinically linked, privacy protected, and transparently documented stands a far greater chance of influencing regulatory outcomes.
Segmed’s Data Framework Translates FDA Guidance Into Practice
At Segmed, we curate large, diverse imaging datasets with standardized metadata to create a traceable, consistent foundation for real-world imaging evidence. Our ingestion pipelines preserve full data provenance, multi-layer quality controls reduce variability, and privacy-preserving mechanisms enable outcomes validation without compromising patient confidentiality. Our RWiD datasets are ethically sourced and support model training, enhancing diagnostic accuracy and generalizability while aligning with regulatory standards for FDA review and broader regulatory use.
With this mission in mind, Segmed provides access to 150M imaging studies from diverse modalities like X-rays, MRIs, CT scans, and ultrasounds. Our regulatory-grade, de-identified, and annotated datasets are ideal for developing AI models across oncology, neurology, and cardiology disease areas. Segmed has been part of more than 35 FDA clearances, multiple foundation models, and fit-for-purpose real-world evidence research projects.
Connect with us to explore how our diverse, high-quality tokenized imaging datasets can enhance the training and validation of healthcare AI models.
Frequented Asked Questions - F.A.Q.
What is real-world imaging data (RWiD) and how does it differ from clinical trial imaging?
Real-world imaging data (RWiD) refers to routine medical images such as CT, MRI, X-ray, and ultrasound scans collected during everyday clinical care. Unlike clinical trial imaging, which follows tightly controlled protocols and selected patient populations, RWiD reflects real-world variability in imaging practices, patient characteristics, and care pathways.
Why does the FDA consider imaging data an important source of real-world evidence (RWE)?
The FDA considers imaging data a key source of real-world evidence because imaging data offers objective, reproducible insights into disease progression and treatment response. When properly curated and standardized, real-world imaging data can support surrogate endpoints, assess real-world effectiveness and safety. Additionally, real-world imaging data can validate AI and digital biomarkers across diverse patient populations.
What does the FDA mean by “fit-for-purpose” imaging-derived RWE?
“Fit-for-purpose” imaging-derived RWE means that the imaging data is appropriate, reliable, and rigorously generated for the specific regulatory question it is intended to address. The FDA expects imaging data to be well-sourced, standardized, transparently analyzed, and clearly linked to clinical context so it can credibly support decisions on safety, effectiveness, or post-market performance.
What are the key requirements for regulatory-grade real-world imaging data?
Regulatory-grade real-world imaging data must have traceable data provenance, standardized acquisition and harmonization, high-quality annotations, validated data transformations, privacy-protected de-identification, strong quality controls, clear linkage to clinical context, and complete documentation to support transparency and audit readiness.
How does Segmed support FDA-aligned real-world imaging evidence?
Segmed supports FDA-aligned real-world imaging evidence by curating regulatory-grade imaging datasets with traceable provenance, standardized acquisition and harmonization, robust quality controls, and privacy-preserving de-identification. Its imaging data is clinically linked, transparently documented, and fit-for-purpose, enabling reliable use in regulatory submissions, AI validation, and real-world evidence generation.
References
- U.S. Food and Drug Administration (FDA). Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices: Guidance for Industry and Food and Drug Administration Staff [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; 2025 Dec 18 [cited 2025 Dec 31]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices
- Alipour-Haris G, Liu X, Acha V, Winterstein AG, Burcu M. Real-world evidence to support regulatory submissions: a landscape review and assessment of use cases. Clin Transl Sci. 2024 Aug;17(8):e13903. doi:10.1111/cts.13903. pubmed.ncbi.nlm.nih.gov
- U.S. Food and Drug Administration (FDA). Framework for FDA’s Real-World Evidence Program [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; 2019 Mar 15 [cited 2025 Dec 31]. Available from: https://www.fda.gov/media/120060/download?attachment
- Sikoglu E. Unlocking the Potential of Medical Imaging as Real-World Data [Internet]. American Pharmaceutical Review; 2023 Jun 1 [cited 2025 Dec 31]. Available from: https://www.americanpharmaceuticalreview.com/Featured-Articles/597636-Unlocking-the-Potential-of-Medical-Imaging-as-Real-World-Data/
- U.S. Food and Drug Administration (FDA). Real-World Data: Assessing Electronic Health Records and Medical Claims Data to Support Regulatory Decision-Making for Drug and Biological Products [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; Jul 2024 [cited 2025 Dec 31]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/real-world-data-assessing-electronic-health-records-and-medical-claims-data-support-regulatory
- U.S. Food and Drug Administration (FDA). FDA Use of Real-World Evidence in Regulatory Decision-Making [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; updated 2025 Sep 26 [cited 2025 Dec 31]. Available from: https://www.fda.gov/science-research/real-world-evidence/fda-use-real-world-evidence-regulatory-decision-making


